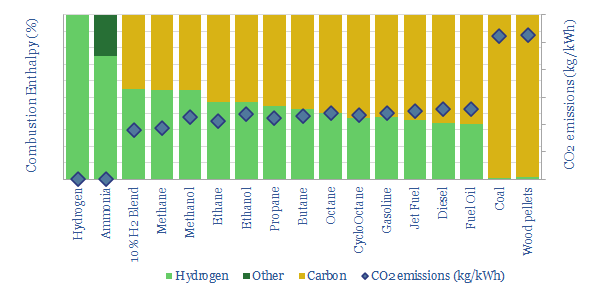
The purpose of this data-file is to disaggregate the energy content of combustion fuels, including natural gas, different oil products, NGLs, coal, hydrogen, methanol, ammonia et al.
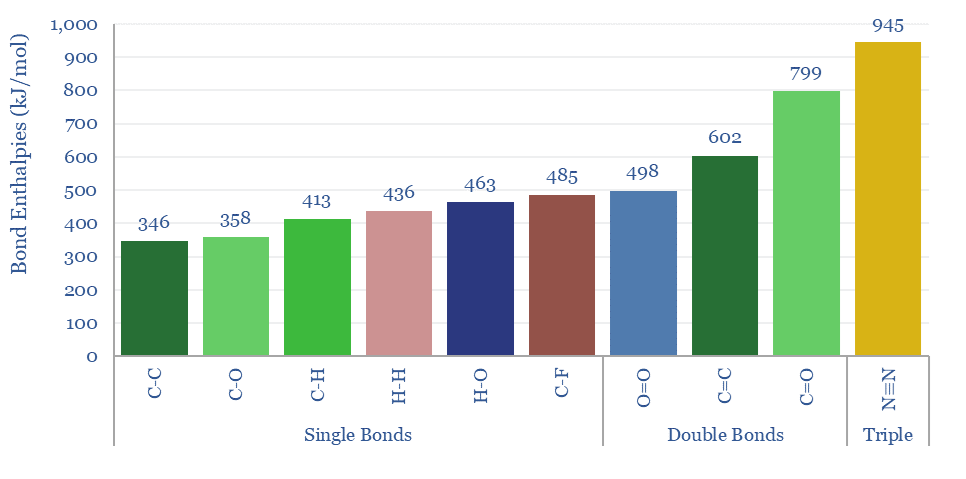
Bond enthalpies denote the amount of energy that can be harnessed when forming bonds, and the amount of energy that is needed to break these same bonds. For example, the reason that many energy-releasing reactions absorb oxygen and produce CO2 is that O=O bonds are relatively weak double bonds at 498kJ/mol, while C=O bonds are relatively strong double bonds at 799 kJ/mol.
In each case, this data-file derives the fuel’s enthalpy of combustion from first principles, the contributing share of carbon and hydrogen oxidation, and thus the CO2 emissions per unit of energy. We also derive the fuels’ energy density, expressed in kWh/kg, kWh/mcf and kWh/gallon.
For example, natural gas combustion releases 850kJ/mol of net energy when combustion, of which c54% derives from hydrogen in CH4 forming water vapor, and c46% derives from the carbon in CH4 forming CO2. Thus methane has an energy content of 304kWh/mcf and a CO2 intensity of 0.19kg/kWh.
Similar calculations are contained in the data-file for hydrogen, ammonia, methanol, ethane, ethanol, propane, butane, octane, gasoline, jet fuel, diesel, fuel oil, coal and wood pellets.
There is recent enthusiasm to lower combustion emissions by blending hydrogen into gas grids. But materially greater decarbonization occurs by replacing coal/biomass with gas, or even replacing oil products with NGLs.
For an overview of bond enthalpy, and how it determines the energy content of combustion fuels, while also forming H2O and CO2, please see our overview of energy units, and our discussion of coal versus gas.