Energy is the glue of our universe. Literally everything is at some level an energy flow – from viewing this text to matter itself – which can be expressed in Joules and kWh. Hence this 16-page overview is a useful reference, to translate from any energy units to any others; for comparisons; and to understand the units in energy transition.
This reference document is an attempt to condense 15-years of energy research into a single overview, for anyone wishing to understand energy from first principles, juggle energy units seamlessly, and compare different energy technologies.
We will consider it ‘mission accomplished’ if this document gets printed, earns its keep on the desks of energy analysts for the foreseeable future; and despite the complex, numerical topic, if it leaves readers in awe of energy and vaguely amused by some of the ‘fun facts’.
Energy is the glue of our universe, and everything that happens within it: making things warmer, cooler, faster, slower, bigger, smaller, visible, audible, electrically charged, or chemically changed. Thus the ‘Joule’ is a miraculous unit, which definitionally links electricity, heat, motion, physics and chemistry, as explained on page 2.
However the kWh is a more useful unit, because 40% of all global energy is electricity, which in turn is primarily metered, bought and sold in kWh (or MWH). Our advice to understand energy transition is to translate everything into kWh (page 3).
An intuitive guide to mastering the orders of magnitude, from kWh to thousands of TWH, is presented on pages 4-5. This includes electrical processes, producing materials and entire countries’ energy balances.
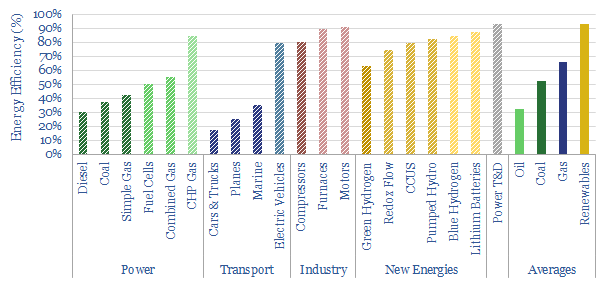
Primary energy versus useful energy is another crucial distinction. Primary energy denotes the thermal energy contained in the bond enthalpy of fuels (yes, we will explain this in a way that is intelligible and avoids unnecessary jargon). But only a portion of this energy can be harnessed as useful energy, due to efficiency losses (page 6-8).
A reference for gas industry units is given on pages 9-10, converting gas units into kWh from standard cubic feet (mcf, mmcf, bcf, TCF), normal cubic feet, cubic meters, tons of gas, tons of LNG (MT, MTpa), British thermal units, the archaic therm; higher heating value versus lower heating value, and real-world gas blends. Gas is a low-carbon fuel as 54% of gas energy comes from converting hydrogen (in CH4) into water vapor.
A reference for oil industry units is given on page 11, converting oil units into kWh from gallons, barrels (bbls, kbbls, Mbbls, bn bbls), boe (boe, kboe, Mboe, bn boe), kilograms and tons; for ethane, methanol, ethanol, propane, butane, octane, gasoline, jet fuel, diesel, fuel oil and the ‘blended average oil barrel’; and the CO2 intensity of oil, of all of the above.
A reference for coal industry units is given on page 12, converting coal units into kWh from tons (kTpa, MTpa, GTpa), and the associated CO2 intensity of coal. The key challenge is understanding different coal grades, from anthracite, to bituminous, to sub-bituminous, to lignite.
A reference for biomass industry units is given on page 13, converting wood fuels into kWh from wet tons, dry tons, and across different products, such as fresh wood, dry wood, deforestation wood, wood chips, briquettes, wood pellets, charcoal.
A reference for food energy units is given on page 14, converting from calories into kWh, for different food products, within the world’s 10GTpa food production. The drawback of the calorie, as a unit of energy is an annoying custom, that many commentators abbreviate kiloCalories as ‘Calories’. This makes many technical papers confusing. It would be as though somewhat decided that ‘dollars’ would be a really handy abbreviation for ‘1,000 dollars’, and then sent you haphazardly into a casino.
A reference for hydrogen units is given on page 15, converting from kg of hydrogen into kWh, both in higher heating value and lower heating value terms, and for different volumes of hydrogen at standard conditions, for liquid hydrogen at -253C, and for compressed hydrogen at 10,000 psi. It is interesting to compare this with natural gas.
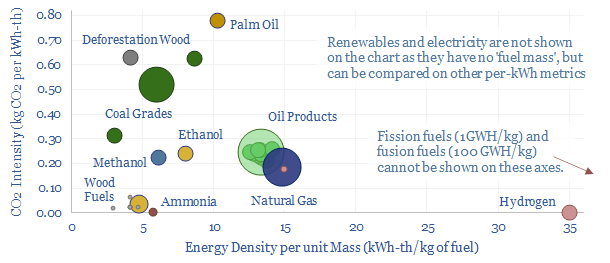
Other fuels and comparisons are also covered on pages 16-17. An amazing fact is that 1 kg of hydrogen releases 3 million times more energy as a fusion fuel than a combustion fuel. We also discuss the first and second laws of thermodynamics, which matters for maximizing the efficiency of clean fuels.
The note ends with some reference tables and links to our other data-files. Another excellent and free resource for comparing different fuels is Engineering Toolbox. We hope our own note is helpful for understanding all of our energy transition research.