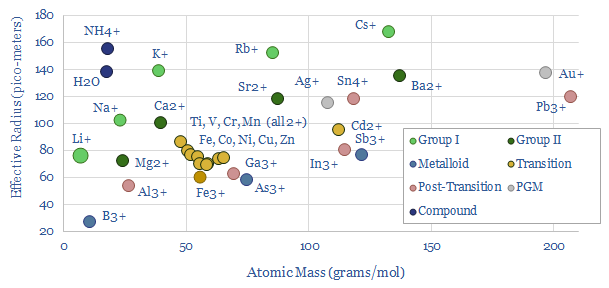
Ionic radius measures the width of ions, in pico-meters (one billionth of a millimeter, one trillionth of a meter), or in angstroms (100 pico-meters). The average across a range of cations is around 100 pico-meters, or 1 angstrom.
But surprisingly, ionic radius is only about 40% correlated with atomic mass. Yes, H+ ions are smallest, at 0.0008 pico-meters, and ionic radius rises as you move ‘upwards’ within each group of the Periodic Table.
But on the other hand, lithium ions (atomic mass = 7) are similar to most transition metal ions (atomic mass 50 – 60), and about 40% larger than aluminium 3+ (atomic mass = 27).
The first reason is that when an atom loses electrons, the remaining electrons become more attracted to the nuclei, and thus cluster in more tightly in 3+ ions than in 2+ ions, which in turn cluster more tightly than 1+ ions.
The relationship is also non-linear for larger atoms, as the added width of wider electron shells is counteracted by adding more protons to the nucleus and thus creating a stronger positive charge that acts on all electrons.
Strictly, for fans of advanced chemistry, ionic radius varies: Ions in a high-spin state will be larger than the same ion in a low-spin state, and ions in solution may behave as though they are even wider based on how strongly and closely they attract a precise number of solvent molecules.
The ionic radius of Rare Earths is also shown in a back-up tab, illustrating the famous lanthanide contraction, which forms the basis for solvent extraction of Rare Earths.
The data-file simply contains the data-behind the chart, in case you wish to have the exact numbers in a useful spreadsheet, or re-format the charts.
Recent commentary: please see our article here. This also informs our outlook on novel battery chemistries, such as CATL’s.