Engines convert heat into work. They are governed by thermodynamics. This note is not a 1,000 page textbook. The goal is to explain different thermodynamic cycles and heat engines, simply, in 13-pages, covering what we think decision makers in the energy transition should know. The theory underpins the appeal of electrification, ultra-efficient gas turbines, CHPs, advanced nuclear and new super-critical CO2 power cycles.
Thermodynamics can be defined as the science governing energy flows. Especially at the “middle levels”, from the kinetic energy of billions of molecules in a container of gas/liquid to the machines, turbines and engines that turn those molecules’ kinetic energy into mechanical work. The three laws of thermodynamics, and the key definitions that every serious decision maker in energy should (probably) know, are summarized on pages 2-3.
Thermodynamic cycles are closed loops whereby different types of heat engines convert heat into work, from a system that starts and finishes with the same internal energy, pressure, volume, temperature and entropy (page 4).
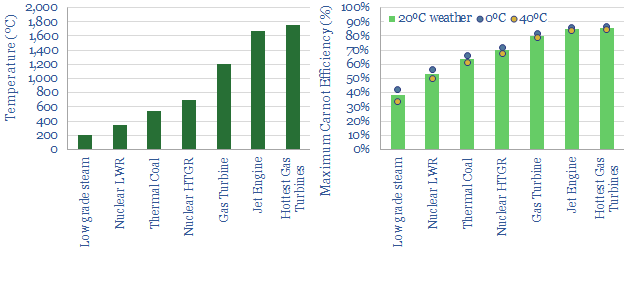
The Carnot Cycle is the most efficient engine in thermodynamics, a physicist’s fantasy, that performs work as it transfers heat from a source to a sink with no losses along the way. The efficiency of the Carnot Cycle equal 1 – TC/TH (both quoted in Kelvin, relative to absolute zero, the coldest possible temperature in our Universe). It follows that the hotter the heat source, the more efficient the engine. Thus the maximum possible (Carnot) efficiency of low-grade steam at 200ºC is 38%, rising to 80-90% from super-heated gas at 1,200-1,750ºC (pages 5-6).
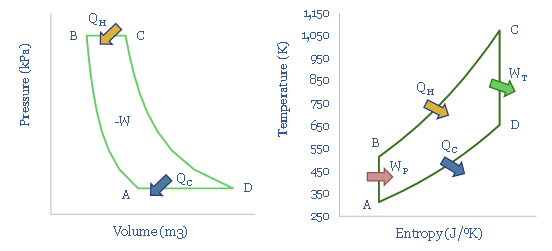
The Rankine Cycle is a modified variant of the Carnot Cycle, which adds a pump and a condenser, and thus approximates the steam engines of the Industrial Revolution, and still in use in many coal and nuclear power plants. There is one great advantage of the Rankine Cycle, and two devastating limitations (pages 7-8).
The Brayton Cycle harnesses work from hot gases, and approximates both jet engines and modern gas turbines. Well-designed Brayton Cycles are always going to ‘beat’ Rankine Cycles. As a rule of thumb, gas turbines are going to be 1.5x more efficient than steam turbines. But they are also harder to engineer, and continue to stretch the boundaries of material science. Indeed, once you grasp the concept of back work ratio, it explains the entire history of the Industrial Revolution and the future role of natural gas in the energy transition. “Gas turbines are better, but steam turbines had to come first” (pages 9-10).
Otto and Diesel Cycles are the thermodynamic cycles used in combustion vehicles’ engines. Theoretical engines are more efficient at higher pressures. But real-world fuels “knock” at higher pressures. This is why relatively more efficient gasoline vehicles rely on high-octane fuels from the refining industry (11-12).
Why do thermodynamic cycles matter in the energy transition? Understanding thermodynamic theory underpins the appeal of electrification, natural gas, ultra-efficient gas turbines, CHPs, advanced nuclear HTGRs and new super-critical CO2 power cycles; and conversely, some relative drawbacks for steam cycles, green hydrogen and electro-fuels.