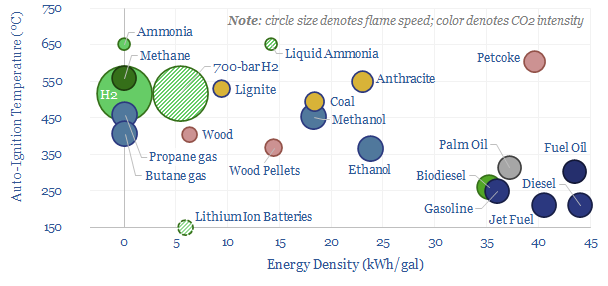
The quality of a combustion fuel comes down to its physical and chemical properties. Hence the purpose of this data-file is to aggregate data into different fuels’ combustion properties, such as their energy content (kg/m3), energy density (kWh/kg, kWh/gal), flash point (ºC), auto-ignition point (ºC) and flame speed (m/s, cm/s). Conclusions about high quality fuels follow.
Gasoline is an excellent transportation fuel. A high energy density of 36 kWh/gal yields a high vehicle range. A low flash point of -40ºC means it is easy to start an engine, even in the dead of winter. A low auto-ignition point of 250ºC means near-complete combustion will occur in an engine cylinder, even one with cold spots. And finally, a high flame speed (0.4 m/s at STP) enables high-RPM engine performance.
Other hydrocarbons have similar properties to gasoline, with high energy densities, low auto-ignition temperatures and high flame speeds.
Natural gas (methane) has the lowest flash point, at -188ºC but one of the higher auto-ignition temperatures of 540ºC, making it well suited to stationary power generation, with fast ramp rates.
Conversely, marine fuel oil has a high flash point, around 85ºC, which limits fire risk. But combustion slip can be an issue in marine engines. Diesel is similar, famously ignited not by a spark plug, but by high pressures in the Otto Cycle.
Solid fuels generally have slower combustion. And more variable combustion conditions, depending on the degree to which they are dried and pulverized.
A typical coal grade might need to be heated above 400ºC to ignite, auto-ignition is at 500ºC, and flame speeds will be 50% lower than hydrocarbons. This makes it slower to ramp up steam engines and steam power plants.
Lower carbon fuels have lower energy density and more variable combustion qualities. Lithium ion batteries have an effective energy density 80-90% below hydrocarbons.
Hydrogen also has low energy density, even when ultra-compressed to 700-bar, while hydrogen also has the lowest flash point of any gas, at -250ºC, explaining a very heavy focus on safety, when we have reviewed hydrogen patent libraries (e.g., NEL).
Ammonia is a possible candidate for a low-carbon fuel, as the combustion of NH3 emits no CO2. Ammonia can be liquid so that its energy density is only 50% below hydrocarbons. But it has one of the highest flash points (130ºC) of any fuel, and one of the lowest flame speeds (80% below hydrocarbons). This creates risks of combustion slip, lower engine responsiveness and the need for a pilot fuel to start up ammonia burners.
Clean methanol is suggested as a better blending alternative to ammonia, as it has a flash point closer to 10ºC and a flame speed similar to liquid fuels (TSE research here).