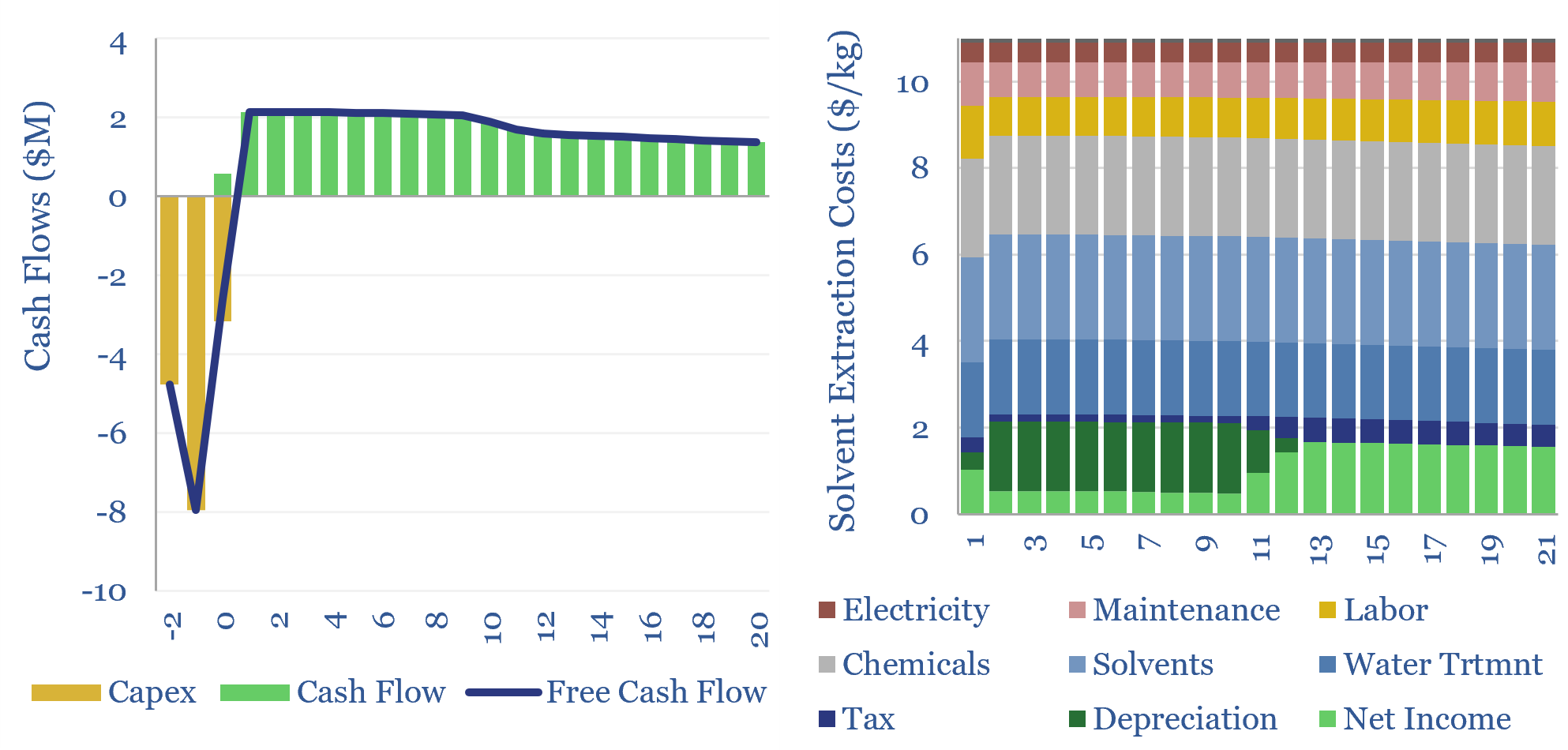
How are Rare Earths separated and concentrated? The process uses solvent extraction in mixer-settlers. It will typically cost over $10/kg, and possibly in the hundreds of dollars per kg. Solvent extraction costs are highly variable and can be stress-tested in this data-file.
Rare Earth metal production starts similarly to any other ore, material is trucked or conveyed to a processing plant where it is crushed, and minerals of interest are concentrated via flotation, then roasted/calcined to change chemical structures, and finally leached in a strong acid solution.
However, the key challenge with Rare Earth metals is that many tend to be found concomitantly, alongside radioactive minerals, and these materials all have very similar properties.
This stems from the very definition of Rare Earth elements as Lanthanides. Lanthanides are the first elements to access 4f orbitals, which is an inner shell, holding up to 14 electrons. They will all happily surrender their 2 outer 5s2 electrons, and one inner 4f electron, before forming stable cations with 3+ charges. Hence they all have very similar chemical properties and cannot be separated, as other metals can, by electrowinning.
However, the 4f orbitals have weak shielding, which means that the nucleus attracts outer electrons more tightly, as atomic number rises, ascending through the lanthanide series, and ionic radius falls (chart below).
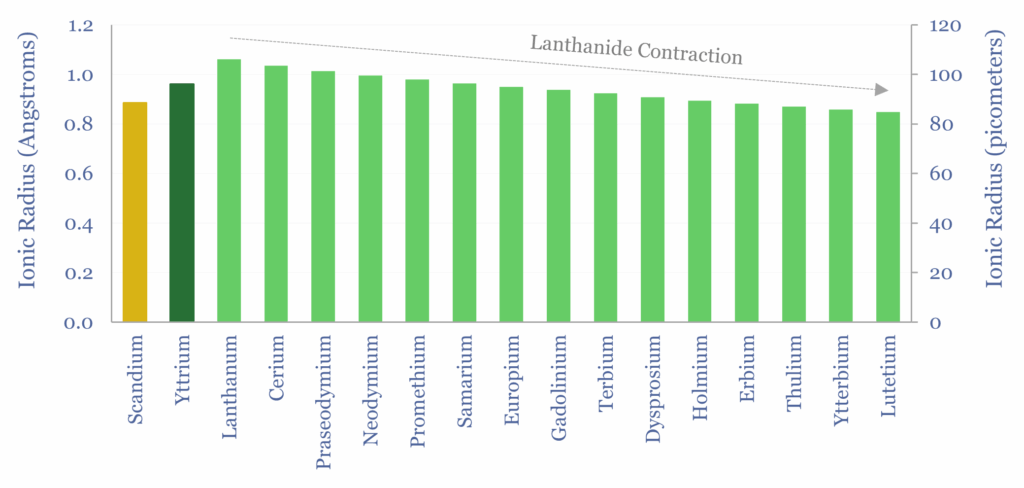
These smaller and mildly more mobile ions will have higher Lewis acidity, and different bonding behaviour with ligands. Hence solvent extraction works via chelation. Chelation is the formation of coordination bonds between metal ions and organic molecules acting as ligands. If one metal bonds more selectively to a chelating agent, then it can be concentrated.
Possible solvents may be based around phosphonic acids, which are derived from phosphoric acid. 2-ethylhexylphosphonic acid mono-(2-ethylhexyl) ester is marketed under the name PC88A, and has been suggested to separate the two neighbors, neodymium and praseodymium, from a mixture of didymium). Syensqo sells a solvent named CYANEX-572, which has been suggested for separating heavy Rare Earths.
Each Rare Earth has its own ideal chemistry, in order to separate it from solutions containing other Rare Earths. The purpose of this data-file is to stress-test how solvent extraction of individual Rare Earths might work, and what it might cost. So few companies produce Rare Earths, and almost exclusively in China, that details are highly opaque.
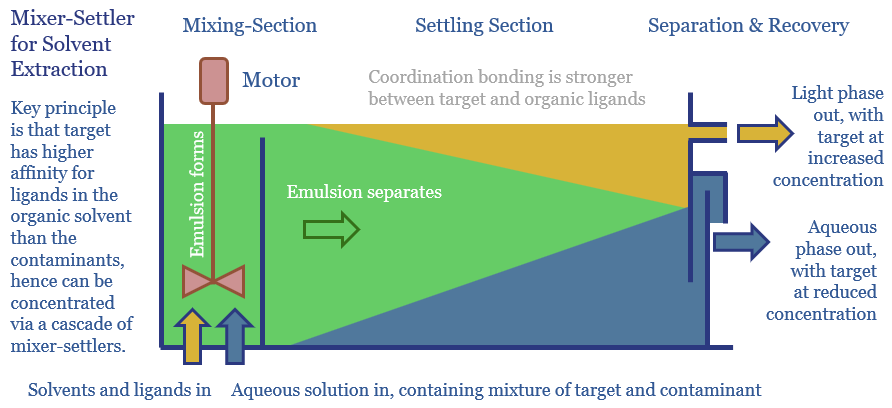
The solvent extraction process for Rare Earths uses mixer-settlers, as depicted below. Organic-soluble ligands are specially chosen which will have a higher affinity for the target Rare Earth. Then an aqueous solution of mixed Rare Earths is introduced into a mixer, agitated by an electric motor, to form an emulsion, which will later settle into a light organic phase (with a slightly higher concentration of the Rare Earth target) and an aqueous phase (with a lower concentration).
Mixer-settlers can then be combined into a cascade, as shown below, with the organic output from each stage flowing through to the next stage, until the organic phase contains a high enough concentration of the target Rare Earth, and the aqueous phase is almost entirely depleted in the Rare Earth.
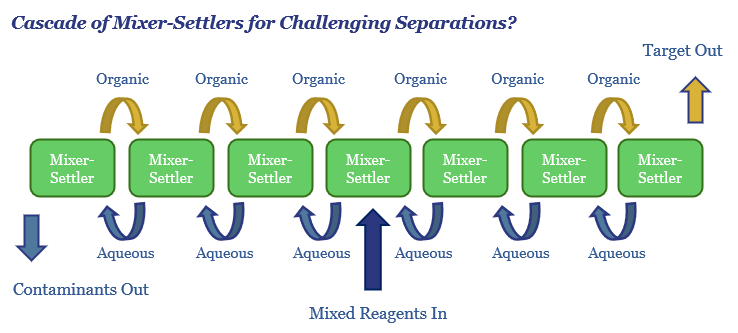
In our data-file, we can model how many mixer-settlers would be needed in a cascade. If 90% of the target metal dissolves in the organic phase, and only 10% of the contaminants do, then a cascade of 7 mixer-settlers, as shown above, should be sufficient to recover 99.9% of the target metal at 99.9% purity (green line below).
However, with Rare Earth separations, it is more likely that c60% of the target metal dissolves in the organic phase at each stage in the cascade, and so does c40% of the contaminant, and thus it takes over 20 stages to recover c97.5% of the Rare Earth of interest at 99.9% purity (pink line below). You can flex these numbers in the data-file.

Solvent extraction costs will therefore depend on the number of stages required, the cost of the solutes, ligand chemicals, water treatment costs prior to water disposal and electricity prices. A sensitivity of costs with the number of stages is illustrated below, but the numbers can vary more widely.
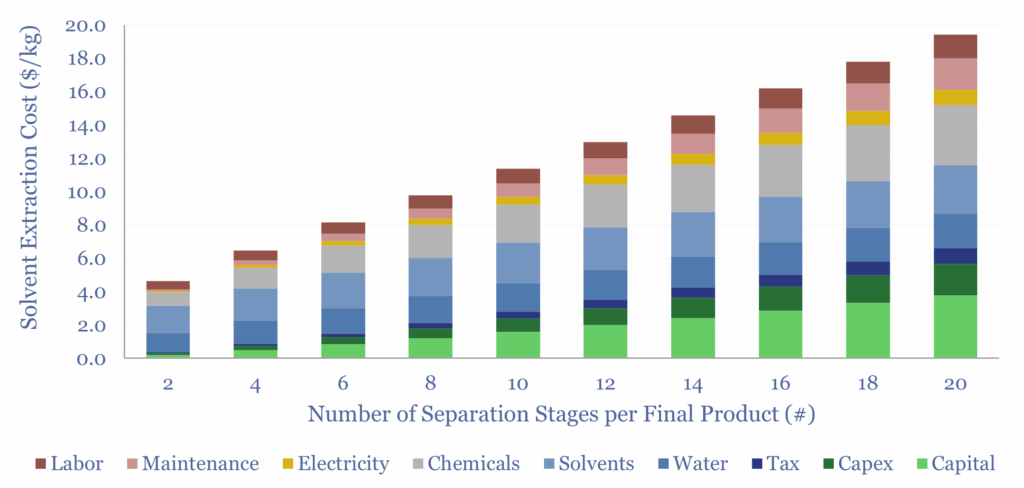
Please download the data-file to stress-test solvent extraction costs, especially for the processing of Rare Earth metals, as a function of these input variables, and see what is needed for a 10% IRR at such a plant.