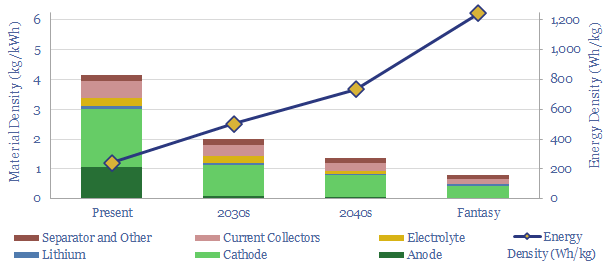
Today’s lithium ion batteries have an energy density of 200-300 Wh/kg. I.e., they contain 4kg of material per kWh of energy storage. Technology gains can see lithium ion batteries’ energy densities doubling to 500Wh/kg in the 2030s, trebling to 750 Wh/kg by the 2040s, and the best possible energy densities are around 1,250 Wh/kg. This is still 90% below hydrocarbons, at 12,000 Wh/kg. Numbers and underlying assumptions are broken down in this data-file.
The energy stored in a battery can be calculated by thinking about the active ions. Active ions are intercalated at the anode when the battery is charged. They surrender electrons to an external circuit. Then these ions diffuse across the electrolyte. Finally, they intercalate at the cathode, where electrons are re-accepted. Thus the energy stored (in Joules) can be calculated by multiplying Faraday’s Constant (in Coulombs per mol) by the cell voltage (in Volts) and the number of mols of ions making this journey from anode to cathode (in mols).
Today’s lithium ion batteries have an energy density of 200-300 Wh/kg. In other words, there is 4kg of material per kWh of energy storage. Of this material build-up, 2 kg is in the cathode, 1 kg is in the anode, 0.6 kg in the current collectors, 0.3 kg in the electrolyte and 0.1 kg in the balance. Different chemistries are assessed in our data-file here.
The maximum energy density of a lithium ion battery can be calculated by increasing the voltage and decreasing the weights of all of the other components. So how much can the energy density of lithium ion batteries improve?
For example, today’s graphite anodes only intercalate 1 lithium ion for every 6 graphite atoms, which weigh 12 g/mol, yielding a charge density of 372 mAh/gram. Silicon anodes weigh more (28g/mol), but they can intercalate 15 lithium ions per 4 silicon atoms, yielding a charge density of 2,577 mAh/gram. (Satisfyingly, these numbers are calculated from first principles in the data-file). So this is an avenue being explored by Amprius, Sila, Enovix. Even denser, could be solid state batteries.
We think the best lithium ion batteries could ultimately reach 1,250 Wh/kg energy densities, although this includes some heroic assumptions and technology gains. It includes a 50% increase in cell voltage, eliminating the anode, eliminating all other excess materials, doubling the charge density of the cathode, thrifting out 90% of the electrolyte and 50% of the current collectors and separators. You can stress test all of these numbers in the data-file.
The biggest uncertainty, in our view, is over cell voltages. Today’s lithium ion batteries run at an average mid-point of 3.6V. Energy density is a direct linear function of voltage. But excess voltages will degrade the battery. For example, 5.9V is the standard potential for decomposition LiF into Li metal and fluorine gas (!).
Could sodium ion batteries have higher energy density? Basic chemistry of the periodic table makes it quite unlikely that any other metal ion could ferry ‘holes’ across an electrochemical cell with the same energy density as lithium. Lithium ions carry a charge of +1 and have a molar mass of 6.94 g/mol. Sodium ions carry a charge of +1 and have a molar mass of 22.9 g/mol.
The energy densities of lithium ion batteries are, in our view, unlikely to surpass 1,250 Wh/kg, on realistic technology pathways, simply based on electrochemistry and simple molar masses, which are broken down in this data-file. This can be compared with the energy density of hydrocarbons and as calculated from bond enthalpies.
Even 200-300Wh/kg energy density of lithium ion batteries justifies the electrification of light passenger vehicles, as electric motors are 2-6x more efficient than combustion engines. But we still see high-density hydrocarbons retaining a major role in aviation, long-distance trucking and shipping. These numbers underpin some of our key vehicle conclusions, hydrocarbon conclusions and battery conclusions amidst the energy transition.