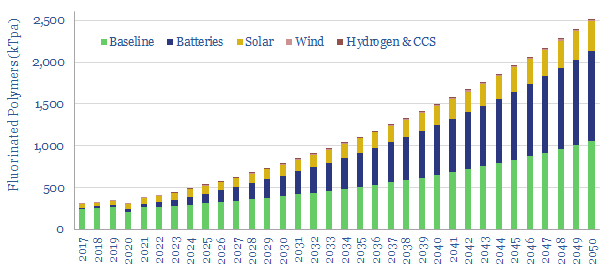
Fluorinated polymers are a ‘stealth bottleneck’ for the energy transition: used in solar back-sheets, battery binders/separators, wind blades, and across the hydrogen chain. But low down the bill of materials, they are easily overlooked. This 400kTpa market grows 6x by 2050. Rising 2021 margins already suggest tightness. And the ‘CO2 curve’ is steeper than any other material. So Western companies must scale up? Our 15-page report explores the upside for fluorinated polymers in the energy transition, and who benefits.
Fluorine is the smallest and simplest halide, with an atomic number of 9 and an atomic mass of 19. C-F bonds have high enthalpy and resistance to thermal and chemical attack. Thus fluorinated polymers are among the most resilient polymers in the world (page 2).
Yet they are overlooked. When you think about the materials in solar panels, you are primarily going to think about silicon, and maybe secondarily silver. When you think about wind, you are primarily going to think about glass fiber, and maybe secondarily resins. When you think about batteries, you are primarily going to think about lithium, and then maybe secondarily graphite or nickel.
Fluorinated polymers are lower on the bill of materials. Yet they are crucial to producing solar panels (page 3), lithium ion batteries (page 4), helpful for wind turbine blade-moulding (page 5) and more broadly in electrification and hydrogen (page 6). In each case, we have estimated future market upside.
Can fluorinated polymers be substituted? We answer this question on page 7, including interesting data on the cracking of solar panel back-sheets. So how are fluorinated polymers produced?
The first stage for producing fluorinated polymers is to mine fluorspar. The key mineral in fluorspar is CaF2. Our outlook for fluorspar markets is discussed on page 8.
The next stage in producing fluorinated polymers is to produce hydrofluoric acid, by reacting acid-grade fluorspar with sulphuric acid, modeled here. Our outlook for hydrofluoric acid is discussed on pages 9-10. Hint: it is used everywhere!
The complicated part is converting methane, chlorine gas and hydrofluoric acid into useful fluorinated polymers. This is one of the most complex value chains we have evaluated in our works. Halocarbon emissions, with GWPs well over 10,000x CO2, can blow the CO2e intensity of these materials out of the water. Some FP product on the market can surpass CO2e intensity of 500 tons/ton, especially for producers that are not focused on environmental credentials (see pages 11-12).
Leading companies in fluorinated polymers, and the upstream value chain, are going to be needed to meet the call for rising future demand, and environmentally minded production processes. Our company screen includes a dozen OECD leaders, and ideas, as discussed on pages 13-15.