Perfluorinated sulfonate (PFSA) membranes, such as Nafion, are the crucial enabler for PEM electrolysers, fuel cells and other industrial processes (e.g., chlor-alkali plants). The market is worth $750M pa. The key challenges are costs, longevity and hydrogen crossover (in mA/cm2), which are tabulated in this data-file.
Nafion was first synthesised by Walther Grot, of E.I. DuPont de Nemours in the 1960s, as a robust cation exchange membrane for the chlor-alkali process, which had previously used materials such as asbestos to separate the anode and cathode sides of the cell.
Today, Nafion’s original patents have expired, and other producers besides Chemours produce PFSA polymers, under various different brand names. But in this article, we will refer to Nafion as a catch-all for similar PFSA membranes.
Nafion turns out to be a remarkable polymer, the enabling membrane for proton exchange membrane electrolysers and fuel cells. It consists of a fluorinated polymer (PTFE) backbone, off of which branch ether groups, connecting to further fluorinated polymers, ultimately terminating in sulfonate groups (SO3H).
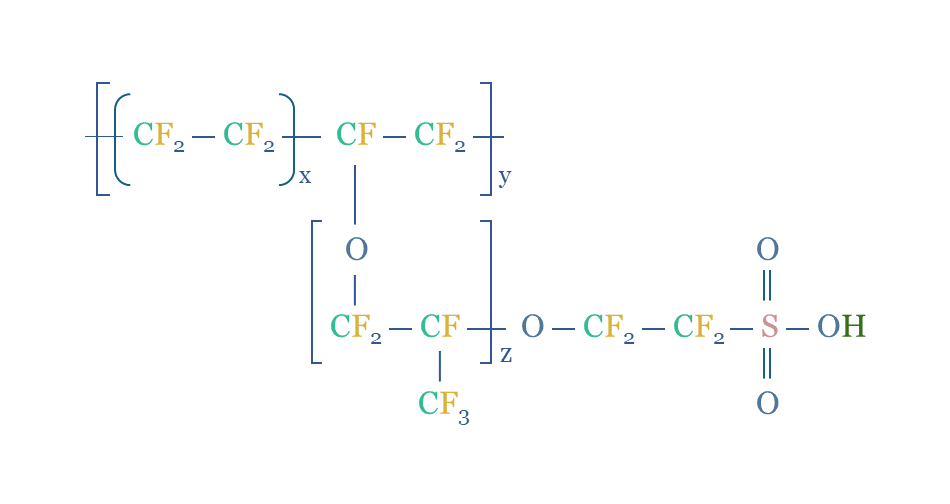
The sulfonate groups are strongly polar, exhibiting surface ultrastructural properties that “appear utterly unlike anything else”. They absorb water and form helical channels of 2-3 nm diameter, through which protons can ‘hop’. So can other small cations. But anions and gases are impeded. This is even the reason that the SpaceX’s Dragon space probe used Nafion membrane to dehumidify air against a vacuum.
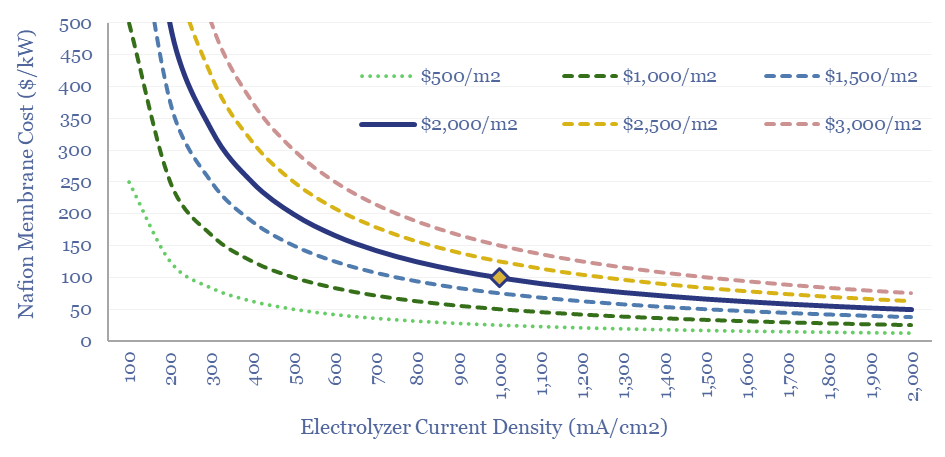
Costs of Nafion membranes are estimated at $2,000/m2, based on data-points from online sources and technical papers. Thus the membranes will comprise $100/kW of cost in an electrolyser at 1,000 mA/cm2. This feeds into our electrolyser cost model, and the numbers can be stress-tested in this data-file.
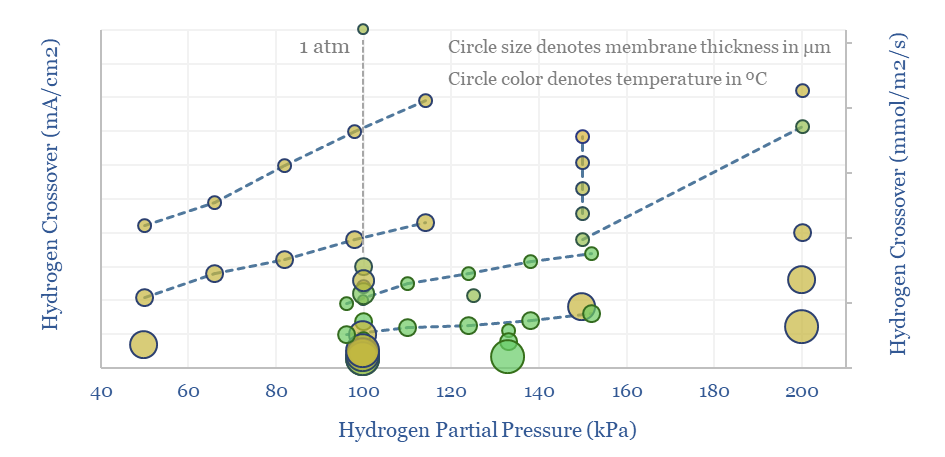
The key challenge with Nafion and other PFSA membranes in a hydrogen electrolyser is hydrogen crossover. For example, this means that H2 forming at the cathode of an electrolyser can diffuse back across the membrane in very small quantities, towards the anode side, and re-oxidize into H2O. This hurts Coulombic efficiency by 0.1-1%.
But the more pressing challenge of hydrogen crossover is that hydrogen oxidation at the electrolyser anode will form not only water, but also peroxide radicals, which then have an annoying habit of degrading catalysts in the anode, the membrane itself, and other cell components.
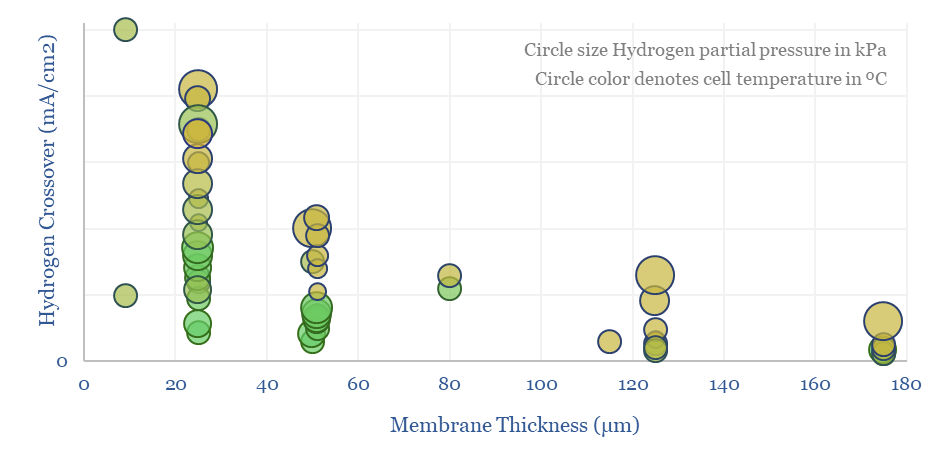
Hydrogen crossover increases linearly with temperature, with H2 partial pressure (itself a function of current density!), for thinner membranes (which have lower resistance and are aimed at maximizing efficiency), and finally with age. Older or degraded membranes have 2-10x higher hydrogen crossover.
Membrane degradation may thus count against putting electrolysers and fuel cells into mobile applications, such as hydrogen cars, hydrogen trucks and planes. For more details, see our overview of electrochemistry and our overview of electrolyser degradation. Details on hydrogen crossover and possible solutions are in the data-file.