Nature based solutions to climate change could extend beyond the world’s land (37bn acres) and into the world’s oceans (85 bn acres). This short article explores one option, ocean iron fertilization, based on technical papers. While the best studies indicate a vast opportunity, uncertainty remains high: on CO2 absorption, sequestration, scale, cost and side-effects. Unhelpfully, research has stalled due to legal opposition.
Nature based solutions to climate change are among the largest and lowest cost opportunities to achieve “net zero” and limit atmospheric CO2 to 450ppm, as summarized here. But so far, all of our research has been limited to land based approaches.
The ocean is much larger, covering 85bn acres, compared with 37bn acres of land. Furthermore, compared to the c900bn tons of carbon in the atmosphere, there is c38,000 bn tons of carbon stored in the oceans (chart below). Of this, c1,000bn tons is near the surface and 37,000 bn tons is in deeper waters. The surface and the deep waters exchange c100 bn tons of carbon per year (in both directions), through the “ocean biological pump”, which is c8x higher than total manmade CO2 emissions of c12bn tons of carbon per annum. These numbers are largely derived from the IPCC and our own models.
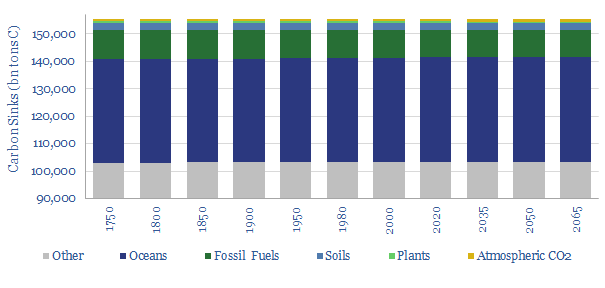
A vast opportunity to mitigate atmospheric CO2 in oceans is suggested by the figures above. The mechanism would need to increase the primary productivity of oceans (i.e., the amount of CO2 taken up by photosynthetic organisms) and the sinking of that fixed organic material into deep oceans, where it would be remain for around c1,000 years.
Below we will describe the process of ocean iron fertilization, which has been explored to sequester CO2 in the intermediate and deep ocean. First, we will introduce some terms and definitions.
An Ocean In Between the Waves
The mixed layer (ML) captures the surface of the ocean. It is named because this surface layer of water is effectively mixed together by turbulence (e.g., waves) so that its composition is relatively homogenous. The depth of the mixed layer ranges from around 20-80 meters. It tends to be larger in the winter than the summer. This is also the layer of the ocean penetrated by light and capable of supporting photosynthesis.
Phytoplankton in the mixed layer are responsible for 40% of the world’s photosynthesis and oxygen production. They are single celled microorganisms that drift through the water. They comprise micro-algae and cyanobacteria. They make up 1-2% of global biomass. Under optimal conditions, algae can fix an enormous 50T of CO2 per acre per year, which is 10x higher than typical forests (data file here).
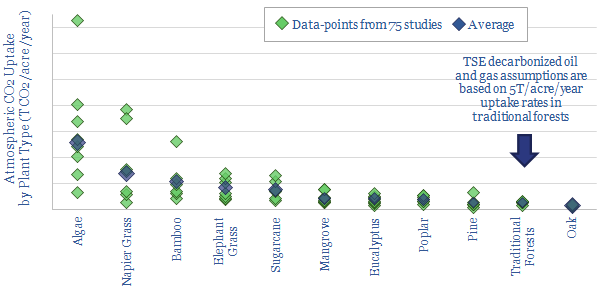
However, typical conditions are not optimal conditions. Total primary productivity of marine organisms is around 100 bn tons per year. This implies CO2 is fixed at around 4T/acre/year, on a gross basis, not including the CO2 that is respired back again by other organisms.
Iron is an essential limiting factor for the uptake of macronutrients in phytoplankton. Typically, with iron concentrations below 0.2nM, phytoplankton cannot absorb macronutrients (especially nitrates) for photosynthesis.
The major source for ocean iron is dust inputs to the ocean from land. Indeed, one theory on the cause of the last Ice Age is a vast uptick in desert dusts or volcanic ash blowing into the ocean, enhancing the productivity of phytoplankton, raising the CO2 dissolved in the oceans, and lowering CO2 in the atmosphere (which was measured at 180ppm at the last glacial maximum, 20,000 years ago, compared to 280ppm in pre-industrial times).
The Martin hypothesis suggests, therefore, that Ocean Iron Fertilization (OIF) could increase oceanic carbon, sequestering CO2 in intermediate- and deep-ocean layers for storage over c1,000-years. As Martin famously (hyperbolically) stated it, “give me half a tanker of iron and I will give you another Ice Age”.
High nutrient low-chlorophyll concentrations (HNLC) indicate the areas where OIF is most likely to be effective. HNLC suggests primary productivity is below potential levels, due to a shortage of iron. HNLC regions include the North Pacific, Equatorial Pacific and Southern Ocean.
Ocean Iron Fertilization: Productivity Increases
6 natural and 13 artificial OIF experiments have been performed since 1990 into ocean iron fertilization, denoted as nOIF and aOIF respectively.
All the aOIF experiments were conducted by releasing commercial iron sulphate dissolved in acidified seawater into the propeller wash of a moving ship, over initial areas from 25-300 sq km. By the end of the experiments fertilized areas have spread as far as 2,400 sq km (as evidenced by sulfur hexafluoride tracers). The iron is rapidly dispersed and taken up, dropping from 3.6nM to 0.25nM in 4-days, and often refertilized.
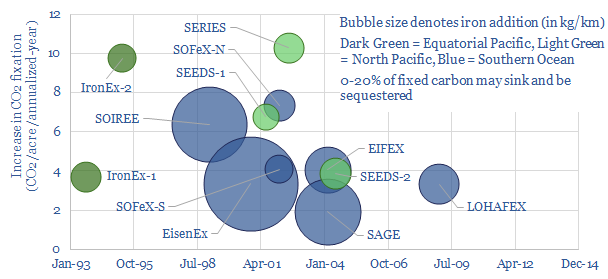
Primary production is significantly enhanced, with potential 100,000:1 ratios of carbon fixation to iron additions. Maximum phytoplankton growth occurs in response to 1.0-2.0nM. For example, in one experiment, denoted as IronEx-2, surface chlorophyll increased 27-fold, peaking at 4 mg/m3 after 7-days, increasing primary productivity by 1.8gC/m2/day. On an annualized basis, this is equivalent to around 10 tons of CO2e per acre per year.
Other studies are shown below. CO2 absorption has been highly variable and does not correlate with the amount of iron that is added. This indicates a complex biophysical system, which requires a deeper understanding.
It’s only a Carbon Sink if the Carbon Sinks.
The largest controversy around the effectiveness of aOIF is whether the carbon will sink into the intermediate and deep oceans. High carbon export has been observed in natural OIF in the Southern Ocean near the Kerguelen Plateau and Crozet Islands, so we know that the process can sequester CO2.
But of the 13 artificial OIF experiments, only one (EIFEX) has conclusively shown additional carbon fixation sinking into the deep ocean. The study saw carbon export down to 3,000m, as phytoplankton blooms aggregated and sank. But others have been less clear cut.
The skeptics argue that across the broader ocean, only 15-20% of CO2 fixed by photosynthesis sinks into the intermediate ocean and just c1-2% sinks into the deep ocean. The remainder is grazed by zooplankton or bacteria, so the fixed carbon is metabolized and respired back into the atmosphere. While CO2 sinking can be higher in nOIF, this is a continuous and slow process, based on the upwelling of iron-rich subsurface waters. Conversely, aOIF will inherently be episodic, with massive short-term iron additions, and thus perhaps struggle to be as effective.
The proponents argue back that past studies have failed to measure carbon sinkage due to limitations in their experimental design. The one clear success, at EIFEX, was a a 39-day study, while others may not have been sufficiently lengthy. In other studies, there were simply no measurements in the deep ocean or outside the fertilized patch for comparison (e.g., IronEx-2). In other studies, the measurement methods over a decade ago may not have been sufficiently — based on tracers (Thorium-234) or physical traps that are meant to collect organic matter, which are known to be disrupted by currents.
Diatom blooms could also enhance future sinkage. Diatoms are a group of unicellular micro-algae that make up nearly half of the organic material in the ocean, forming in colonies that tend to aggregate and sink more readily than other phytoplankton types. Primary productivity has doubled in past aOIF studies where diatoms dominated. The prevalence of diatoms in phytoplankton blooms can be enhanced in areas rich in silicates.
Future experiments can also test the process more effectively, identifying the right conditions for diatoms to dominate the blooms, aggregate and sink; which in tun hinges on abundant silicates and low grazing pressure from mesozooplankton. It is suggested to conduct studies in ocean eddies, which naturally isolate 25-250km diameter areas for 10-100 days. More precise measurement is also possible using satellite data; and unmanned aquatic vehicles equipped with transmissometers, which measure the impedance of light by materials such as sinking organic matter (our screen below finds a rich improvement in autonomy and precision of concepts for the oil and gas industry).
Unintended climate consequences and feedback loops?
The other criticism of OIF is that interfering with nature ecosystems can have unintended consequences, both for biodiversity and for climate.
N2O is a complication. It is a 250x more potent greenhouse gas than CO2. The ocean is already a significant source of N2O, from bacterial mineralization. N2O increased by 8% at 30-50m during on aOIF trial, named SERIES. Models suggest excess N2O after 6-weeks could offset 6-12% of the CO2 fixation benefit. Conversely, other studies suggest OIF acts as a sink for N2O, as it also sinks alongside aggregates.
Dimethyl Sulfide (DMS) is another by-product of aOIF, from the enzymatic cleavage of materials in planktons. DMSs may be a precursor of sulfate aerosols that cause cloud formation. This would counteract global warming. Fertilizing 2% of the Southern Ocean could increase DMS c20% and produce a 2C decrease in air temperatures over the area, one study has estimated. Others disagree and do not find increases in DMS from aOIF.
A commercial hurdle: commercial aOIF is currently illegal
The current legal framework actually prohibits OIF in international waters because of a perceived threat of environment damage by profit-motivated enterprises. Specifically, regulations from 2008 and 2013 categorize OIF as marine geo-engineering and thus it is not allowed at large scale (>300 sq km) or commercially.
This seems unhelpful for unlocking a potentially material solution to climate change. Companies such as GreenSea Venture and Climos, which were set up to harness the opportunity appear to have dissolved. As one recent technical paper stated, “no other marine scientific institutions are willing to take up the challenge of carrying out new experiments due to the fear of negative publicity”.
Others have illegally explored OIF, flouting regulations. For instance, in 2012, Haida Salmon Restoration dumped 100 tons of iron sulphate into international waters off Haida Gwai, British Columbia, in an attempt to raise salmon populations.
Conclusion: large potential, large uncertainty and likely stalled
The costs of OIF are highly uncertain and estimates have ranged from $8/ton of CO2 to $400/ton of CO2. It is currently not clear how a commercial aOIF project would need to be designed in order to calculate precise costs.
Total CO2 uptake potential from ocean iron fertilization is also vastly uncertain and has been estimated between 100M and 5bn tons of CO2 per year globally. The upper end of the range could be conceived as c0.5T of CO2-equivalents sinking per acre per year across a vast c10bn acres of ocean. But again, this is not possible on today’s understanding.
The technique is likely limited to oceans that are deficient in iron but rich enouch in other nutrients (e.g., the North Pacific, Equatorial Pacific and Southern Ocean). Moreover, blooms are limited to c2-months over summer, where nutrients are welling up from subsurface waters, light is available but grazing pressure from zooplankton remains light.
Uncertainty is very high and for now the technique is stalled due to stifling regulation and low research activity. Hence for now, we reflect OIF on our CO2 cost curve, but we have taken the more conservative ranges above as inputs.
Sources:
Yoon, J-E., Yoo, K-C., MacDonald, A., et al (2018). Reviews and syntheses: Ocean iron fertilization experiments – past, present, and future looking to a future Korean Iron Fertilization Experiment in the Southern Ocean (KIFES) project. Biogeosciences, 15.
Ciais, P., C. Sabine, G. Bala, L. Bopp, V. Brovkin, J. Canadell, A. Chhabra, R. DeFries, J. Galloway, M. Heimann, C. Jones, C. Le Quéré, R.B. Myneni, S. Piao & P. Thornton, (2013). Carbon and Other Biogeochemical Cycles. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Report to Congress (2010). The Potential of Ocean Fertilization for Climate Change Mitigation.