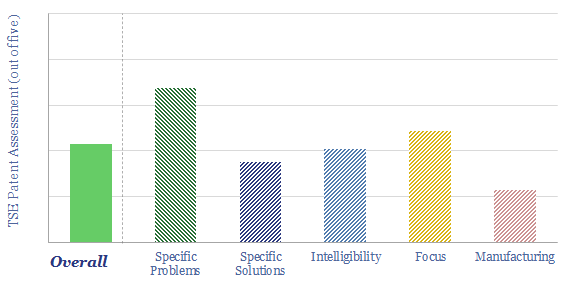
Boston Metal is a private company, spun out of MIT in 2012, developing “a revolutionary one-step process to decarbonize steel production using clean electricity”, around 4MWH/ton of steel. This data-file is a Boston Metal technology review, based on assessing 55 patents across 3 families. We were unable to de-risk the technology simply based on these patents. A key focus area is conveying current into the MOE cell, as it operates around 1,600C, which is above the melting point of most conductor materials.
The global steel industry emits over 2 tons of CO2 per ton of steel (model here), as it produces 2GTpa of steel materials (steel research here), which equates to total CO2 emissions of 4GTpa, or 8% of the global total (data here). We think a key challenge for decarbonizing steel is to avoid large inflation in steel costs, which would cascade through to inflate the costs of substantively all new energies (note here).
Boston Metal is aiming to commercialize a molten oxide electrolysis process, using large currents to reduce the Fe3+ ions in molten Fe2O3 into pure iron, which can be tapped from the bottom of the cell.
An industrial analogy comes from the Hall-Héroult process, which dissolves alumina (Al2O3, 2,000C melting point) in molten cryolite (Na3AlF6, at 1,000C), then applies a large electrical current to reduce aluminium metal (model here). The beauty of this system is in the cryolite. Not only does it halve the operating temperature of the cell, but the sodium cations (-2.71V) have a standard electrode potential that is more negative than the aluminium ions (-1.67V), so the aluminium ions will preferentially reduce into metal while the sodium will remain ionized (more here).
The key challenge for MOE of iron ore is temperature. From our patent review, we could not identify any clear candidate solvents, which would materially lower the melting point needed for electrolysis of iron oxide. Boston Metal also alludes to its cells running at 1,600C, which is near the melting point of iron/iron ore.
What materials could serve as current collectors for these cells? Most conductive metals, and even most super-alloys, have melted by 1,500C. There are refractory ceramics that tolerate temperatures around 2,500C, but they are not conductive. There are refractory metals that tolerate these high temperatures, but they have higher redox potentials than iron, meaning they would oxidize and cease to be conductive? Likewise, graphite has a very high melting point, but is prone to oxidizing into CO2 at such high temperatures.
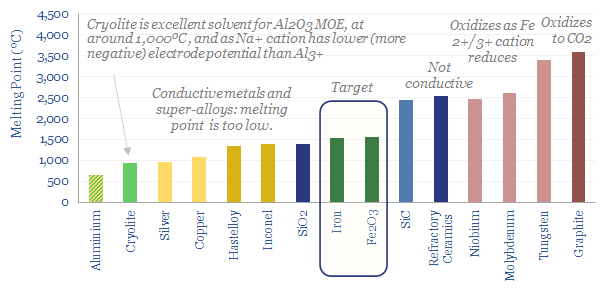
The Boston Metal patents that we reviewed addressed the challenge of designing current collectors that would melt without excessive convective mixing with the molten iron. This is achieved in one patent by optimizing their geometry.
We also need to acknowledge that Boston Metal may have developed technologies that are not covered in their patents. And our patent reviews always involve some guesswork. However, we hope the work is constructive and helps decision makers to understand what has been IP-protected, and to guide future analysis and questions.
Please download our Boston Metal technology review, for our observations about these patents, their specificity, focus and scale-up into commercial manufacturing; and how these patents compare to other patent libraries that we have assessed.