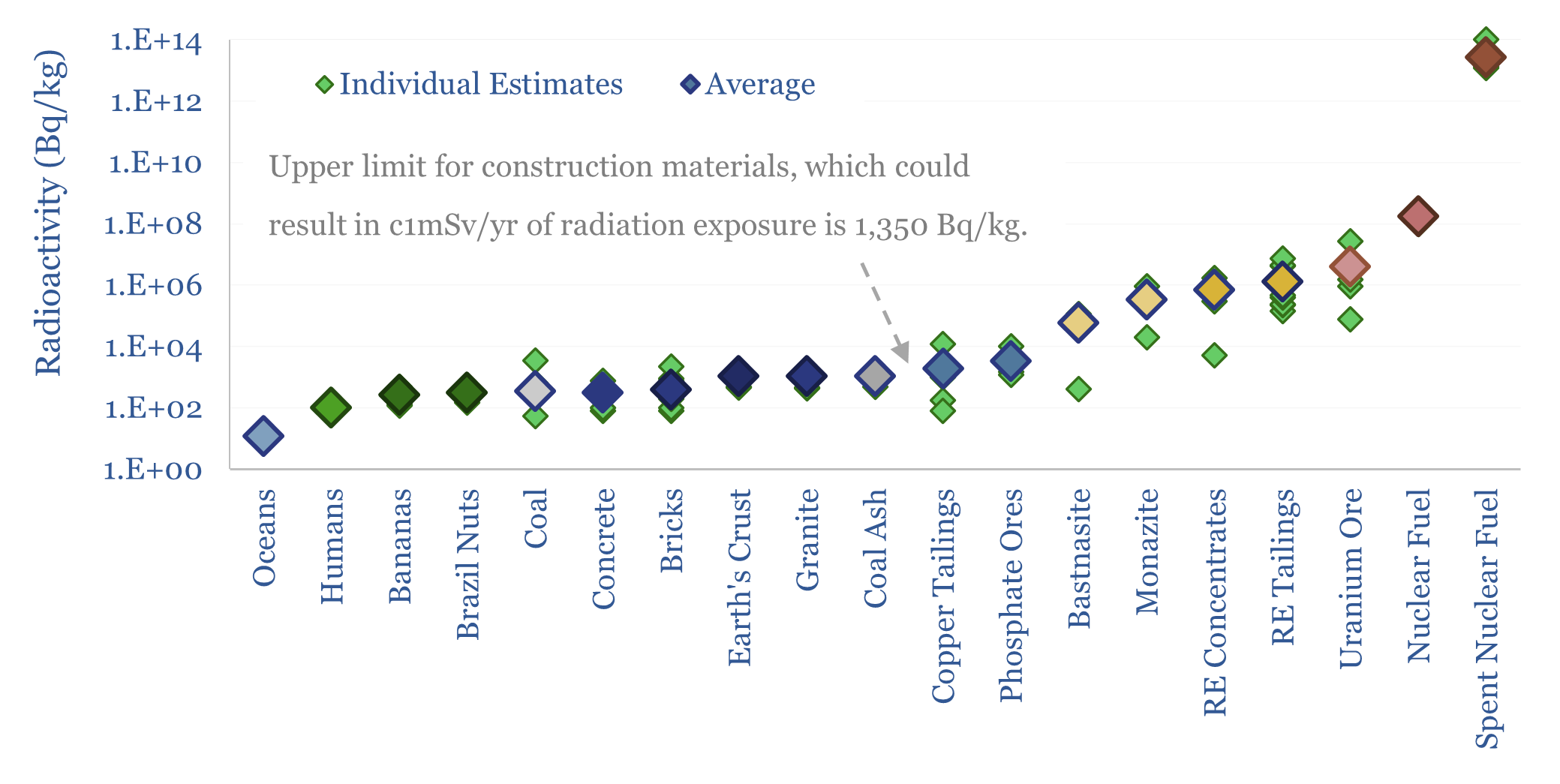
This data-file captures the radioactivity of materials: ranging from 10Bq/kg in sea-water, to 100Bq/kg in humans, to 300Bq/kg in Brazil nuts (the most radioactivity food), to 1,000Bq/kg in coal ash, 2,000Bq/kg in copper tailings, 0.5-1MBq/kg in Rare Earth concentrates and tailings, to 4M Bq/kg in uranium ore, 0.2 bn Bq/kg in nuclear fuel and 10-100 trn Bq/kg in spent nuclear fuel.
One Becquerel (1 Bq) denotes the radioactive decay of one atomic nucleus per second, which will in the process emit alpha particles (a helium nucleus), beta particles (an electron or a positron), gamma rays (electromagnetic radiation), or less commonly, protons and neutrons.
Radioactive exposure in humans is measured in Sieverts. 1 Sievert = 100 rem. 1 Sievert denotes that 1 Joule of energy from radioactive decay has been absorbed per kg of tissue. The average person gets 2.4 millisieverts of natural radiation per year, which is derived from proximity to – especially inhalation and ingestion of – radioactive materials.
Major radionuclides on Earth include Potassium 40, whose decay into Argon-40 is responsible for the 1% argon in the Earth’s atmosphere, as accessed commercially by air separation plants. Carbon-14 makes up 1.25 atoms per trillion carbon atoms, whose presence can be used to date organic materials. Radon-222 originates from the decay of Radium-226 and ranges from 4-20Bq/m3 in outside air, but can build up to higher levels in unventilated rooms. Uranium-238 and Thorium-232 are also naturally occurring.
Ocean water is one of the least radioactive substances in our data-file below, which plots different estimates of the radioactivity of materials, and then takes an average. The hydrogen and oxygen in water have very low radioactivity, while there is maybe 10Bq/kg of radioactivity derived from dissolved salts.
Humans are themselves very mildly radioactive, at around 100Bq/kg, containing around 60Bq/kg from Potassium-40 in their bones, and 40Bq/kg of Carbon-14 in organic tissue.
Brazil nuts have one of the highest radioactivities of any food, estimated at around 300 Bq/kg on average, mainly from Potassium-40 and Radium-226. Eating 1-2 Brazil nuts per day might result in 100-200 μSv/year of radiation exposure.
Building materials such as concrete and bricks typically have 300-400 Bq/kg of radioactivity, which will lead to inhabitants of those buildings enduring <0.5 mSv/year of radiation exposure. Building codes prohibit using materials with over 1,350 Bq/kg of radioactivity, which might lead to over 1mSv/year of radiation exposure for inhabitants. So this is a good rule-of-thumb to keep in mind.
Earth’s crust is more radioactive again, over 1,000 Bq/kg, of which half derives from Potassium-40 at 3ppm, and smaller amounts come from Radium-226, Thorium-232 and Uranium-238. It is the ongoing radioactive decay of material in the Earth’s subsurface that causes the average geothermal gradient of 20-30ºC/km, as exploited in enhanced geothermal.
Coal ash is the world’s largest naturally occurring radioactive material (NORM) waste stream, at 300MTpa. Coal contains 3ppm Uranium in Australia, 13ppm in Germany, 20ppm in China. Thorium concentration is 3x Uranium. And these elements get concentrated 5-10x more in the ash, to 500-2,000 Bq/kg. Thus, without scrubbing and filtering exhaust gases, the radioactivity from burning coal would actually be higher than from the entire nuclear industry according to the World Nuclear Association.
The mining industry is often trying to obtain heavier atoms that can be commercially extracted from the Earth’s crust. These atoms co-occur with radionuclides. Thus there are reports of 2,000-10,000 Bq/kg radioactivity in some copper tailings, or phosphate ores.
Rare Earths are particularly challenging in the mining industry, as the heavy lanthanides, comprising elements 57-71, often naturally co-occur with Uranium and Thorium, in ores such as bastnasites and monazites. During the mining process, all of the heavier components are crushed into powders then concentrated by flotation. So concentrates and tailings can be 5-10x higher than in ores, and reach 1M Bq/kg, albeit varying by +/- 1 order of magnitude, case by case. Safe disposal of these radioactive wastes requires vitrifying them in glass, encasing them in concrete, and/or burying them underground. Many policymakers often talk about the desire to re-shore Rare Earth supply chains to the West, but it does require handling radioactive concentrates and waste products.
The nuclear industry itself handles ores with 1-25M Bq/kg of radioactivity, based on uranium ore grades ranging from 0.6% in the US to 17% at Cigar Lake. This uranium is concentrated, then enriched in the key fissile isotope of Uranium-235. The fission of each Uranium-235 atom releases 202MeV of energy in a nuclear reactor. It also releases three neutrons, which convert Uranium-238 into Plutonium-239, and leads to a cocktail of highly radioactive isotopes. Thus, nuclear waste has the highest radioactivity of anything in our screen, at 10-100 Bq/kg and requiring safe disposal.