Coal provided 25% of the world’s primary energy in the past three years, but 40% of all global CO2 emissions. Gas also provided 25% of the world’s primary energy but just 15% of the CO2 (data below). In other words, gas’s CO2 intensity is 50% less than coal’s. The purpose of today’s short note is to explain the different carbon intensities from first principles.
Explanation #1: half the energy in gas is from hydrogen
Burning coal releases energy as carbon is converted into CO2. In other words, substantively all of the energy from coal combustion is associated with CO2 emissions.
Burning gas releases energy as methane (CH4) is converted into CO2 and H2O. In other words, just over half of the energy from gas combustion is associated with innocuous water vapor, and just less than half is associated with CO2 emissions.
This is simple chemistry. For many decision-makers, this chemistry is sufficient to explain why switching all of the world’s future potential coal energy to gas energy can directly underpin one-fifth of the decarbonization on realistic roadmaps to net zero (note below). For others, who want to get into the nerdy details of bond enthalpies, we have written the note below.
Explanation #2: bond enthalpies?
If you wish to delve deeper into the numbers behind gas and coal’s CO2 intensities, then our discussion below will help you understand the thermodynamic calculations. As an ongoing reference, the numbers are also spelled out in our bond enthalpy data-file.
Bond enthalpies. Atoms are bonded together into molecules. ‘Bond Enthalpy’ denotes the total thermodynamic energy that is contained in each of these bonds (data here), as determined by fundamental electromagnetic forces that define the universe (note here). In other words, bond enthalpy is the minimum amount of energy that must be supplied in order to dissociate the atoms on either side of the bond; and the maximum amount of energy that could be harnessed when these atoms bond together.
Bond enthalpies are often quoted in kJ/mol. As a reminder, 1 Joule is the energy transferred when a force of 1 Newton acts over a distance of 1 meter; or when 1 Watt of power is exerted for 1 second; or when a current of 1 Amp flows through a resistance of 1 ohm. And 1 mol is a standard for counting the numbers of atoms or atomic reactions, described 6.022 × 10²³ units. This precise number, in turn, was chosen so that 1 mol of protons would have a mass of 1g, and all larger molecules would have an atomic mass that effectively matches their atomic number of protons and neutrons.
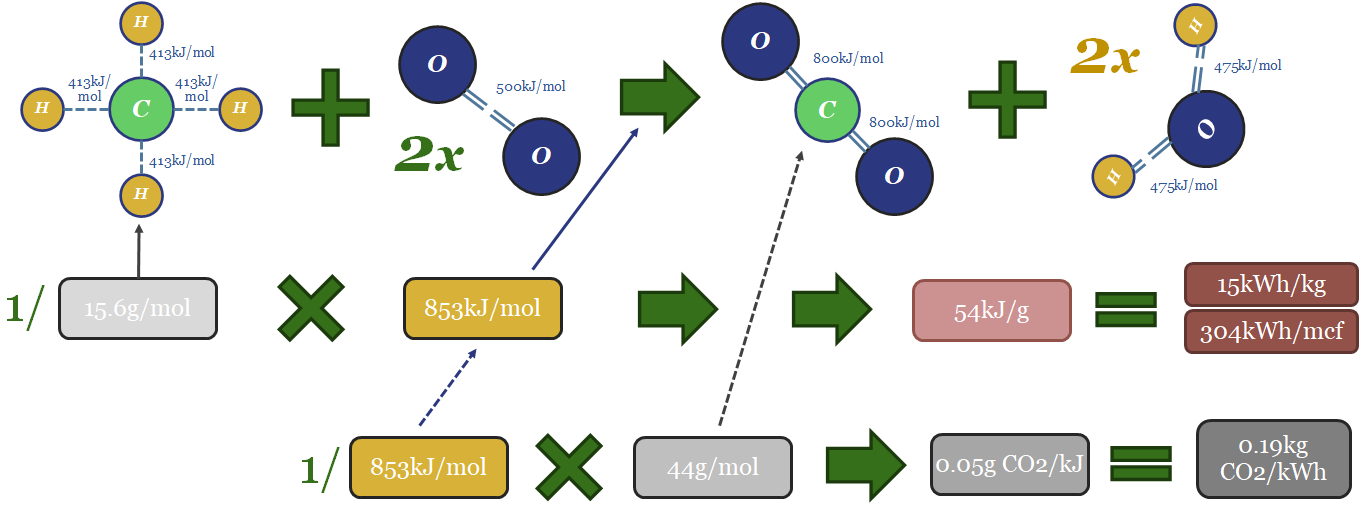
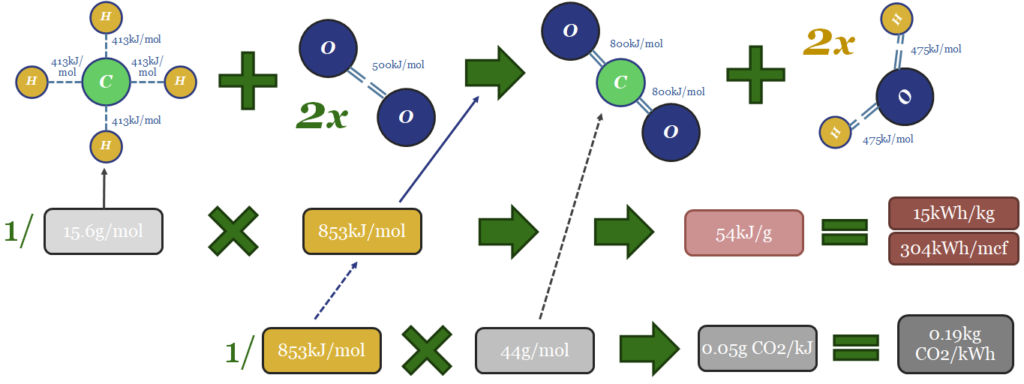
Thus the thermodynamics of gas can be computed from bond enthalpies in the image below. Breaking the bonds in the methane molecule requires 1,652 kJ/mol of input energy. Breaking the bonds in 2 x O2 molecules requires 996kJ/mol. Total bond-breaking energy is 2,648kJ/mol. On the other side of the equation, forming the bonds in 1 CO2 molecule releases 1,598kJ/mol. And forming the bonds in 2 x H2O molecules releases 1,903kJ/mol. Total bond-making energy is 3,501kJ/mol (of which 54% is from forming water molecules). Subtract 2,648 from 3,501, and the result is 853kJ/mol of total energy being released. 1 kJ = 0.2778 Wh. So with some unit juggling, we arrive at 15kWh/kg of energy generation, or 304kWh/mcf of gas (at 48.7mcf of gas per ton; or 48.7bcf per MTpa for those who prefer LNG units).
The CO2 emissions will include 1 mol of CO2 per mol of methane. That mol of CO2 weighs 44 grams. Hence if you divide 44 grams by 853kJ, the result is 0.05 g of CO2 per kJ. Divide by 0.2778kWh/kg and the result is 0.19kg of CO2 per kWh. Multiply by 304kWh/mcf and the result is 56kg of CO2/mcf.
Likewise the thermodynamics of coal can be computed in the same way. Forming each mol CO2 from C and O releases releasing 1,598kJ/mol. That side of the equation of the easy. Next, if the coal was perfect, pure carbon then the energy that would need to be supplied for bond breaking would be 50% x 4 x C-C bonds at 346kJ/mol (692kJ/mol total), plus 1 x O=O bond (498kJ/mol), for a total bond-breaking energy of 1,190kJ/mol. But in practice, we assume that coal is usually only 80% carbon, while remaining impurities include water (which must be evaporated off), sulphur, nitrogen and other ashy impurities. It will vary grade-by-grade. But on average we think 300kJ/mol is a sensible assumption for the energy release. This yields some important conclusions…
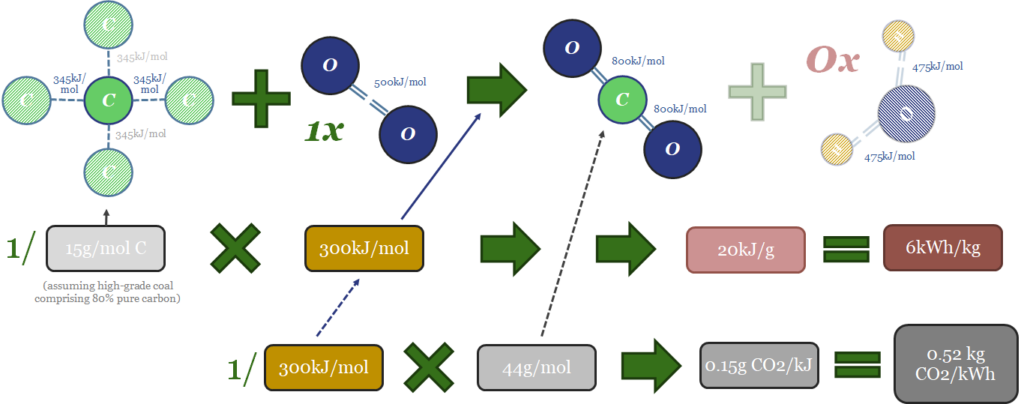
(a) 300kJ of energy is released when 1 mol of coal combustion occurs. This is 65% less than when 1 mol of gas is burned. The main reason, as stated above, is that the coal combustion reaction does not generate any energy from producing water vapor.
(b) 20kJ/gram or 6kWh/kg of energy is released per unit mass of coal consumption. This is c60% less than when an equivalent mass of methane is burned.
(c) Minimal extra mass, as we assume methane weighs 15.6g/mol, versus coal at 15g/mol of combustible carbon (pure carbon is 12g/mol, but we assumed high-grade coal has only 80% carbon). To re-iterate, this means that 1 kg of natural gas is generating 2.5x more energy than 1kg of coal. Again, the reason comes down to hydrogen atoms in methane, which generate 54% of the energy release when they are oxidized to H2O, but in a very dense package of mass. At 1g/mol, hydrogen atoms are much lighter than carbon atoms at 12g/mol and oxygen at 16g/mol. (The hydrogen industry is currently looking for the perfect hydrogen carrier — is it ammonia? is it toluene? — in our view, a near-perfect one already exists, it is called natural gas, and it comes straight out of the ground).
(d) 1 mol of CO2 is released when 1 mol of coal is combusted. This is the same as the amount of CO2 released when 1 mol of gas is combusted. But to re-iterate gas combustion generates around 2.5x more energy.
(e) CO2 intensity can be as high as 0.5kg/kWh for coal combustion. Again this is 2.5x higher than gas combustion, and we have derived the result that gas provides the same amount of energy as coal despite emitting 60% less CO2. There is nothing here except maths and science.
Explanation #3: advanced thermodynamic considerations?
We have glossed over some important thermodynamic concepts in our explanation above. For completeness, we address them here. Those who are bored of abstruse academic details can probably skip ahead to the next section.
Strictly, the useful energy that can be obtained from combusting a fuel is not a pure function of bond enthalpies. You must also deduct a small amount for the change in entropy (Gibbs Free Energy = Enthalpy – T-Delta-S). We have not considered entropy changes in our numbers above. Neither coal nor gas combustion increase entropy by increasing the number of molecules in circulation. But both coal and gas combust with a flame temperature around 1,950C, which is going to increase the entropy of their surrounding thermodynamic systems and prevent their full bond enthalpies from being harnessed.
Another issue is higher versus lower heating values. Specifically, our schematic above showed the combustion of methane releasing 54kJ/g of energy, via the formation of CO2 and H2O. However, 5-10% of this ‘gross calorific value’ energy that is released will be lost in the water vapor. Water is a liquid at ambient temperatures and pressures. Vaporizing that water into an exhaust gas will absorb some of the energy from the combustion reaction. The amount depends on the atmospheric conditions. This is why textbooks quote the ‘net calorific value’ of methane closer at 50kJ/g at standard conditions of 0C and 1-bar. Vaporizing water is not an issue for coal combustion as there is effectively no water produced in that reaction. This narrows the ‘energy gap’ between gas and coal in practice.
Another issue is that our bond enthalpies for coal above were not quite right. We used the average bond enthalpies for Carbon-Carbon single bonds. But the carbon in coal may contain ring structures, aromatic compounds, unsaturated bonds, and particles that are not chemically bound together at all. All of this will most likely lower the bond enthalpies within coal. So our numbers for coal combustion enthalpy are imprecise, and probably a little bit too conservative. Moreover, the rocks found out in the world that we call “coal”, tend to physically contain other hydrocarbons, such as methane, within them. Overall, real world coal grades come in closer to 0.37 kg/kWh (data below).
Another issue is that ‘coal’ is a broad term, covering a range of different fuels, with different carbon contents and different impurities. These will vary. One useful online resource, suggests that energy content can range from 32.6kJ/g for the highest-grade pure anthracite coals, through to 30kJ/g for bituminous, 24kJ/g for sub-bituminous and 14kJ/k for lignite. In 2020, the average ton of coal produced in the US had a grade of 19.8 mmbtu/ton, equivalent to 5,800kWh/tonne, or 23kJ/g. This is probably a bigger issue for energy density per kg than it is for CO2 emissions per kWh.
Finally, coal may be moderately less likely to combust completely, producing small portions of soot and carbon monoxide, especially when burned in small-scale furnaces. This will detract from both the energy content and has a debatable impact on CO2 credentials.
(1) What about emissions across the supply chain?
One potential issue with the numbers we have presented above is that we also need to consider the CO2-equivalent emissions from the supply chains of producing gas and coal, respectively. If, for example, the emissions of producing natural gas were materially higher than the emissions of producing coal, then we would need to factor this in.
However, our analysis finds that often the total full-cycle emissions footprint of producing and distributing coal (usually 50kg/boe or higher) will be similar or higher than the emissions footprint of producing and distributing gas (10-60kg/boe). The free note below gives a full overview of the data we have reviewed here.
(2) What about efficiency of combustion?
A second potential issue with our analysis could be if it were easier to extract the energy from coal than from gas. For example, capturing 80% of the energy from a 0.52kgCO2/kWh fuel would result in lower emissions than capturing 20% of the energy from a 0.2kgCO2/kWh fuel.
Yet again, the data we have reviewed points to higher combustion efficiencies on gas. Our models for a coal-fired power plant assume c40% efficiencies, while our models of combined cycle gas plants average 57% efficiencies, and we are particularly excited about emerging gas-fired CHP systems that can reach 80-90% total thermal efficiencies (note below).
(3) What about ease of carbon capture and offset?
A third factor that is worth considering is the ease of capturing the carbon from combusting coal and gas. We think there is nothing wrong with continued fossil energy use in a fully decarbonized global energy system, as long as the CO2 emissions from that fossil energy is fully captured or offset.
Across our work, we find there are mixed opportunities and challenges for integrating CCS with coal and gas, but it is 2.5x easier to integrate gas with nature-based carbon removals, because there is 60% less CO2 that needs to be offset in the first place.
CCS momentum has also stepped up impressively in the past year (notes below). Coal combustion might seem to have a natural advantage, as its CO2 exhaust stream tends to be c10% concentrated, versus 4% for gas combustion. However, we also find gas’s exhaust CO2 can be concentrated towards 10% by combustion technologies such as exhaust gas re-circulation, gas benefits from fewer impurities that can poison amine cocktails, emerging technologies such as blue hydrogen can decarbonize gas at source, and there are also practical ways of blending gas back-ups with renewables in fully decarbonized power grids (notes below).
(4) What about the costs?
The dimension that has most kept coal burning in the world’s energy mix is its absurdly low cost. A new mine requires $60/ton for a 10% IRR, equivalent to producing thermal energy for 1c/kWh (model below). Natural gas can actually beat this cost, as the best gas fields are economical below $1/mcf (0.3c/kWh), and we estimate that $2/mcf pricing can support passable IRRs in the shale industry (model also below). But on top of this, global gas value chains can bring delivered cost to $6-8/mcf (2-3c/kWh). The biggest challenge, we find, is that starving gas value chains of capital may have re-inflated marginal costs to $12-16/mcf (4-5c/kWh) (third note below).
Conclusion: coal to gas switching cuts CO2 by 50%
The conclusion across our analysis above is that each TWH of energy that is generated from gas rather than coal will result in 50% less CO2, which will lower the burden that is placed on other decarbonisation technologies in our roadmap to net zero. Stated another way, each MTpa of LNG that is developed will likely go on to obviate 5MTpa of CO2 emissions.
So far in the energy transition, however, our depressed observation is that ideological fantasies may have delayed the implementation of real, low-cost and practical CO2 reductions, such as coal-to-gas switching. We think this may change in 2022, as energy shortages deepen (note below), and the world needs more pragmatic options, to accelerate its path towards net zero. Our lowest-cost roadmap to net zero by 2050 requires global gas output to rise by 2.5x.