The electrical conductivity of energy transition materials is tabulated in this data-file, intended as a useful reference.
Electricity conductivity is simply the inverse of electrical resistivity, measured in Ohm-meters, and varying from 10^-30 in super-conducting materials through to 10^20 at the most highly insulating plastics as might feature in HVDC power cables.
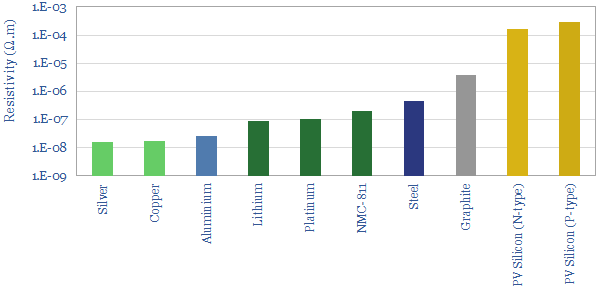
Most of ‘the action’ in energy transition will take place in the range of 10^-8 to 10^-3 Ohm-meters.
Silver is the most conductive metal used in the energy transition, which combined with its high stability, to make it the most commonly used front contact material in solar cells, which in turn consume around 10% of global silver production today.
Copper is used in machinery and appliances. As a rule of thumb, wind, solar and EVs are around 4x more copper intensive than the conventional generators and ICE vehicles they replace. Hence we see copper demand trebling in the energy transition.
Aluminium is c50% more resistive than copper, but it is also 70% lighter and stronger, which explains why it is used in overhead transmission lines or in rigid conductors behind the back contact of solar panels.
Battery metals are 5-10x more resistive, and graphite is 200x more resistive, than the excellent conductors discussed above, because they are primarily selected for their ability to intercalate lithium ions and promote battery energy density.
Electrical grade steel is another 3x more resistive again versus battery metals. Electrical grade steel is used in electric motors, transformers and generators, in order to create electro-magnetic fields.
Finally, PV silicon is a semiconductor, around 10,000 more resistive than conductive metals, because in order to conduct electricity, it requires electrons to be promoted from their valence bands to their conductance bands. Conductivity depends on doping levels (silicon metal hardly conducts at all) and it is higher for N-type silicon the P-type silicon.
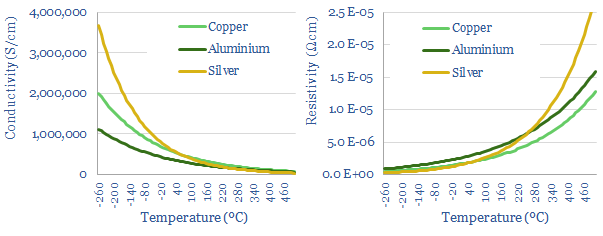
This data-file simply gives some ballpark estimates for the resistivity of different materials. For metals like copper, conductivity tends to fall by -0.4% per 1C of temperature, as higher temperatures cause atomic nuclei to vibrate more, which in turn causes ‘lattice scattering’, impeding the mobility of electrons (charts below and data in the file). This ‘lattice scattering’ is why power grid losses rise on hot days, or why ‘high temperature superconductors’ are a rarity.
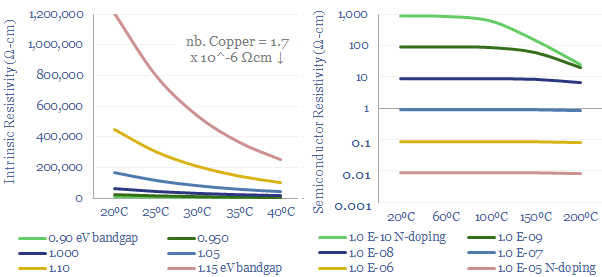
For semiconductors, conductivity is particularly complex, varying primarily as a function of doping. Unlike metals, intrinsic semiconductors become more conductive at higher temperatures, because additional energy helps to promote valence band electrons into conduction bands where they can conduct an electrical charge. We have built up a separate datafile calculating semiconductor conductivity from first principles.
To read more about The electrical conductivity of energy transition materials, please see our article here.