Hot potassium carbonate is a post-combustion CCS technology that bypasses the degradation issues of amines, and can help to decarbonize power, BECCS and cement plants. We think costs are around $100/ton and energy penalties are 30-50%. Potassium carbonate CCS can be stress-tested in this data-file, across 50 inputs.
Potassium carbonate (K2CO3) is a safe, abundant and low-cost salt that can absorb CO2, as soluble CO3(2-) ion reacts with H2O and CO2 to form 2 x HCO3(-) ions. The rich solution can then be steam-treated to re-release pure CO2, forming a CCS process.
Potassium carbonate has been used at over 600 hundred natural gas sweetening plants historically, removing small quantities of acid gases from pressurized gas streams (e.g., in the range of 20-bar) (aka the Benfield Process).
The great advantage of potassium carbonate CCS over the amine process is that there are no toxic breakdown products. This may be particularly helpful when the combustion source is burning waste, biomass/BECCS or cement plants.
The disadvantage of potassium carbonate CCS is that the reaction between CO2 and K2CO3 is slow. For more context see our overview of CCS absorber units. Thus realistic plant designs require higher temperatures (80-100ºC) and pressures (12-20 bar). This can create large energy penalties for potassium carbonate CCS, quantified herein.
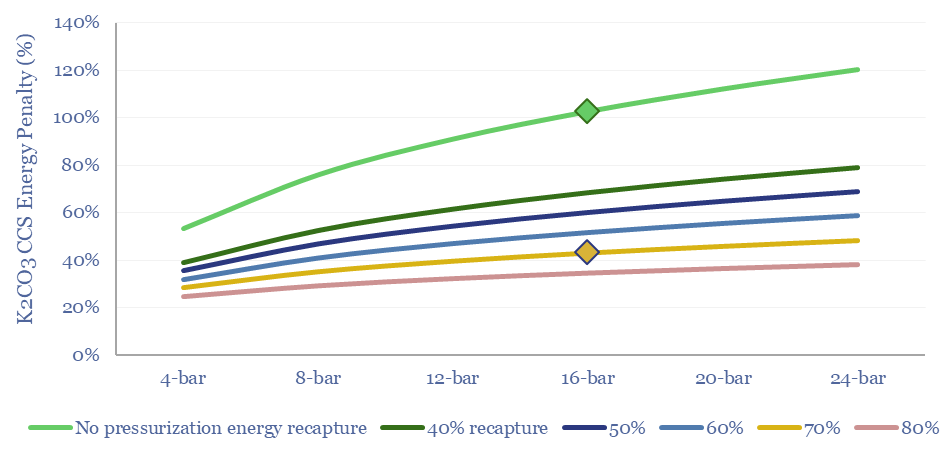
What energy penalties for K2CO3 CCS? If there is only 4-12% CO2 in the exhaust gas of a boiler or burner, then compressing the entire exhaust stream towards the middle of this range can use up 65-90% of the useful energy released by combusting the fuel.
The best option to lower the energy penalties is to re-capture the energy of exhaust gas compression. This is achieved by re-expanding these compressed and CO2-depleted gases back across a turbine (which may directly drive the input compressors; for more background, please see our overview of thermodynamics and CCS energy penalties).
Capsol Technologies (formerly known as Sargas) is listed in Norway and has filed patents for variations of this process running back to 2003 (tabulated in the data-file). It is currently developing what could be “Europe’s first large-scale negative emissions plant” capturing the CO2 from a bio-energy plant in Stockholm.
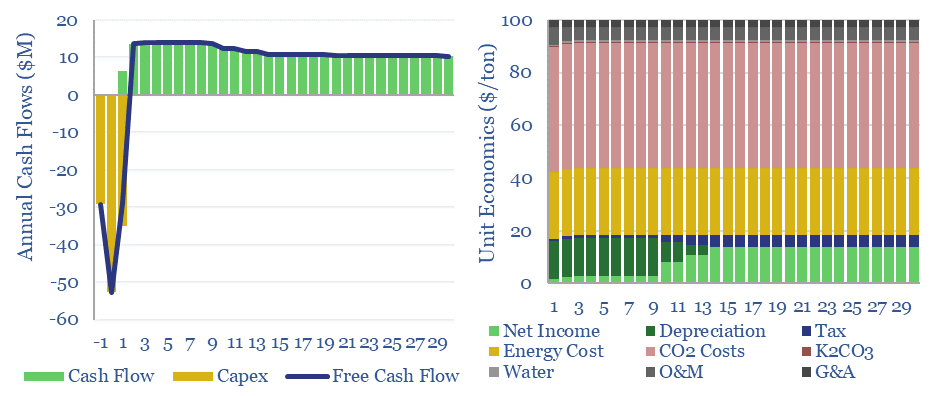
What energy economics for Capsol Technologies’ process? We have read some of Capsol’s patents, its claims of pressure recapture and steam-recirculation, and can simplistically model how this would impact costs and energy penalties (quantified in the data-file in $/ton, in % energy penalty terms, in kWh/ton or GJ/ton, and in kg/kWh CO2 intensities).
Others have looked to reduce the requisite pressurization energy for potassium carbonate CCS by blending K2CO3 with amines (often piperazine). But this seems to defeat the rationale for using potassium carbonate in the first place, which was to avoid emissions of amines or their toxic breakdown products.
Another interesting option could be exhaust gas recirculation, to boost CO2 concentrations and lower compression loads. In some configurations oxygen blending can further lower the volumes of gases that need to be compressed and cover the energy costs of oxygen generation in an air separation plant.
This data-file allows you to stress-test the energy penalties for potassium carbonate CCS in percentage terms, in kWh/ton, GJ/ton and to compute the resultant CO2 intensity of generated electricity in kg/kWh.