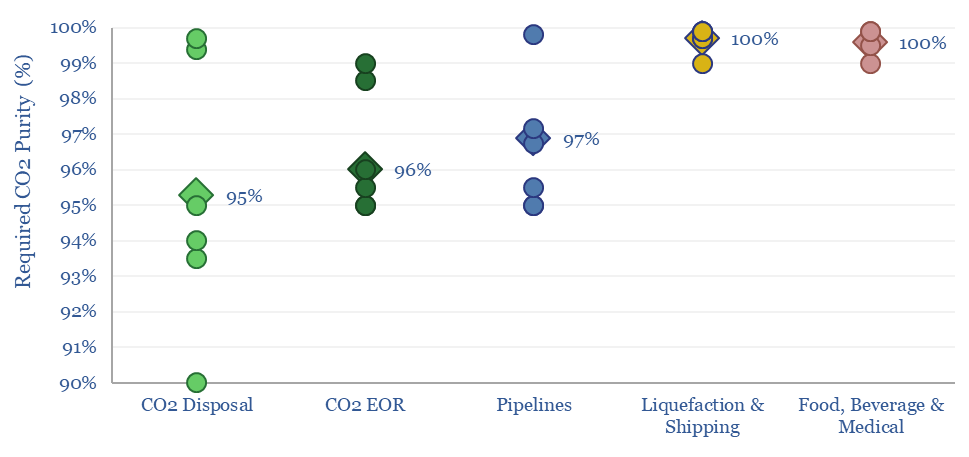
The minimum CO2 purity for CCS starts at 90%, while a typical CO2 disposal site requires 95%, CO2-EOR requires 96%, CO2 pipelines require 97% and CO2 liquefaction or shipping requires >99%. This data-file aggregates numbers from technical papers and seeks to explain CO2 purity for transport and disposal.
Our roadmap to net zero includes 7GTpa of CO2 disposal, across various technologies, from straight-run amine CCS, to DAC, CO2-EOR, blue hydrogen SMRs and ATRs, oxy-combustion, potassium carbonate, other sorbents, next-gen membranes. But what CO2 purity levels do these technologies need to meet?
Energy efficiency is the first reason that CO2 purity matters. As a very simple rule of thumb, compressing a gas stream to 80-200 bar requires 90-120 kWh/ton of compression energy. If the gas stream is only 90% CO2, then the energy costs per unit of CO2 are around 10% higher.
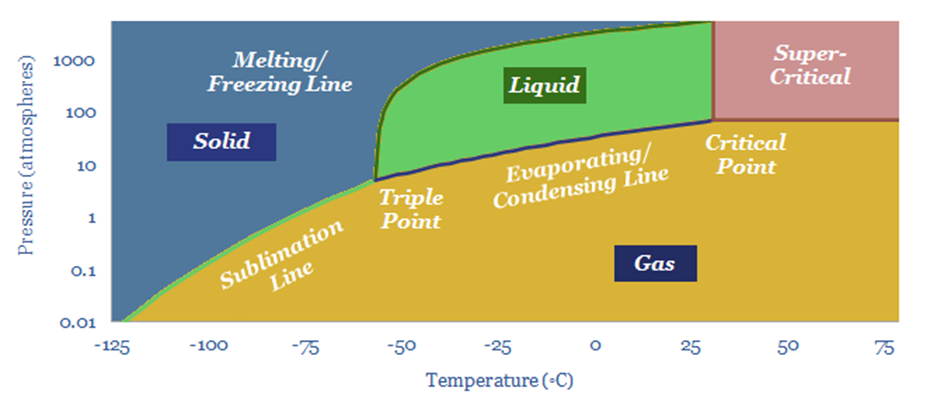
Or more. The reason it is necessary to compress CO2 to >80-bar is so that the CO2 will transition into a dense (super-critical) phase. The phase diagram below shows the critical point for pure CO2. But impurities require higher pressures before CO2 reaches supercriticality.
Larger pipelines are also required to move larger quantities of gas at higher pressures. This matters because larger pipelines with thicker walls have higher capex costs, per our data-file into gas pipeline costs.
The other key reason that CO2 purity matters for CCS is that if the gas stream has less than 100% CO2, then by definition, it must contain something else. Clearly, issues will arise is the ‘what else’ is toxic or hazardous (e.g., H2S, amine degradation products such as nitrosamines, NOx, SOx, etc). But even innocuous contaminants can have an impact.
Water is a key impurity that must be managed in a CO2 pipeline. If puddles of water precipitate out, then they will slowly start dissolving CO2, and greatly accelerate pipeline corrosion. CO2 + H2O -> H2CO3 (carbonic acid). H2CO3 -> 2H[+] + CO3[2-]. Fe(s) + 2H[+](aq) -> Fe[2+] (aq) + H2 (g). It is never good to dissolve your pipeline from the inside out. Furthermore, the H2 can cause further stress cracking.
Hence water is usually limited to <500ppm, ideally <50ppm. This is more of a convention than a hard rule (examples are tabulated in the data-file). Usually, as much as 4,450 ppm of water will be soluble in pure CO2 at 40◦C and 100-bar pressures. Even with 10% nitrogen impurities, this only reduces to 3,400 ppm. Some amine breakdown products, or NO2 can have a more “dramatic effect” on the width of the phase envelope.
But there is also always a margin of safety for cold spots, bends in the pipeline or in the case of depressurization. A pipeline operator has the prerogative to set whatever standards it deems necessary to protect the longevity and efficiency of its investment. Off-spec CO2 may be charged a materially higher transportation tariff if it is accepted at all.
CO2-EOR also requires a higher purity than straight-run CCS, in order to promote miscibility of the CO2 with oil in the subsurface, which will help to swell and mobilize it. This is less important for simple geological disposal.
CO2 transportation by ship or CO2 transportation by truck also requires very high purity, well above 99%, in order to liquefy the CO2, at 20 to -50◦C and 7-15 bar pressures. For example, any residual water vapor in the stream is going to freeze out and plug the system. So this requires the highest purity of CCS value chains and dedicated dehydration.
CO2 purity for CCS will generally need to be above 95%, and ideally will be as high as possible. This favors CCS technologies that can create highly concentrated CO2 streams from exhaust gases of differing CO2 concentrations. But the limits are not overly strict, or likely to deter CCS, in our view. For more related research, please see our overview of CCS value chains.